
Inward remittance of the country shrinks by 23%

VC Niaz Ahmed Khan resigns

One dies while attending Janaza!

Bangladesh will not join ICC T20 World Cup in India
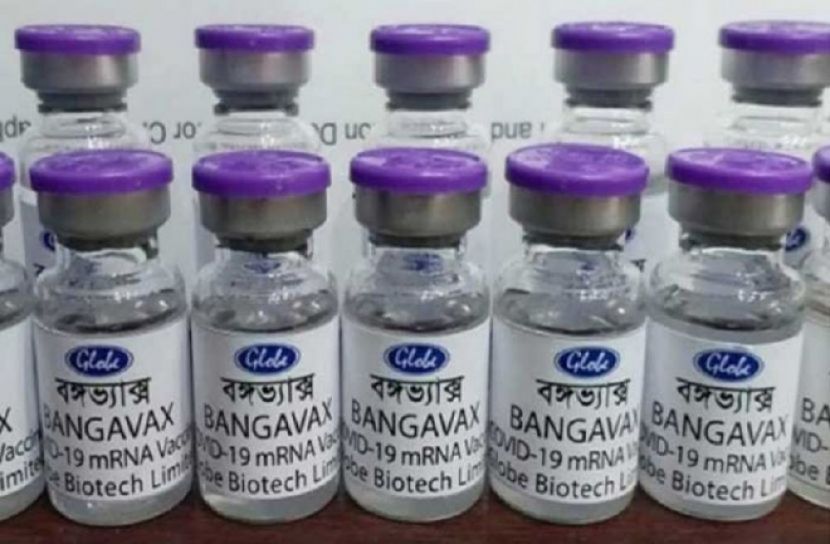
Staff Reporter: The government has given policy approval to the human trial of Corona vaccine Bangavax prepared by Globe Biotech Limited.
The Bangladesh Medical Research Council (BMRC) gave its approval on Tuesday (November 23) after the trial of the Bangavax ticker was successful in animals.
BMRC director, D. Ruhul Amin confirmed the matter.
He said that permission has been granted for testing Phase-I (first phase). Written permission has not yet been granted. The rest will be looked after by the Department of Drug Administration.
On the other hand, the senior manager of the quality and regulatory department of Globe Biotech Limited. Mohammad Mohiuddin said that Globe Biotech will start the experimental application in human body as soon as it gets the approval of the Department of Drug Administration.
"We will apply as soon as possible," he said. After making any vaccine, you have to go through various tests step by step. In the latest experimental application in the human body, it is recognized as a vaccine after seeing the success of making antibodies against certain diseases.
Newsletter
Subscribe to our newsletter and stay updated.


